Cervical cancer web-based clinical decision support tool
Home Back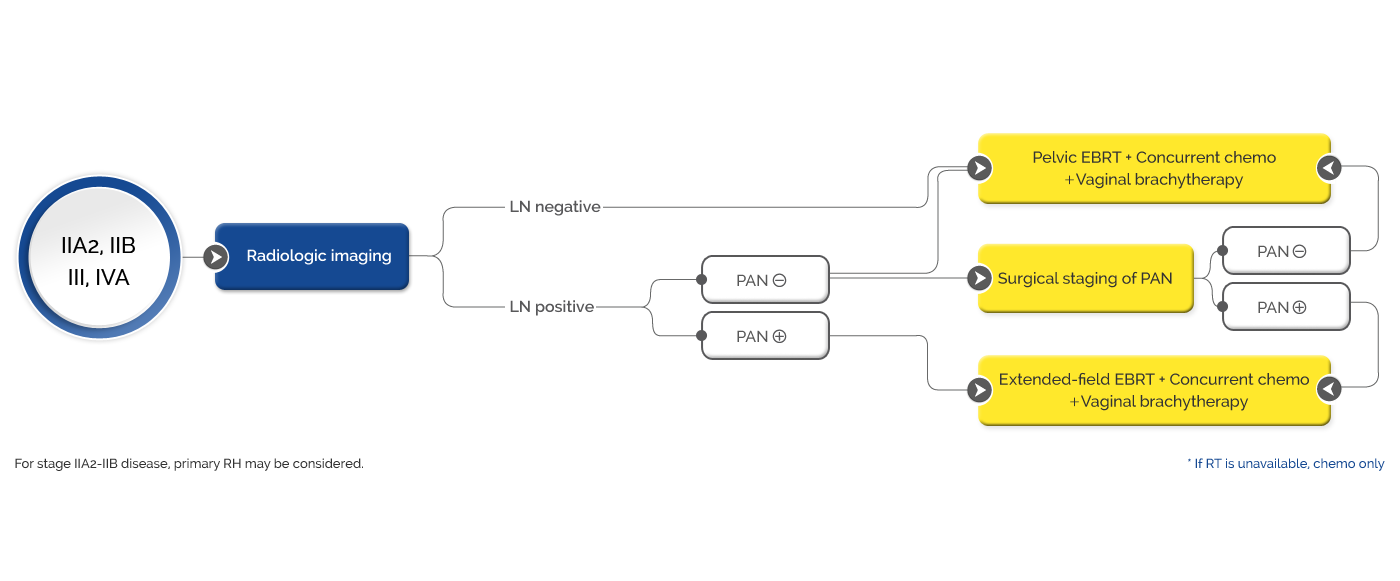
- Based on the results of phase III ENGOT-cx11/GOG 3047/KEYNOTE-A18 trial, addition of an immune checkpoint inhibitor to primary CCRT can be an option.
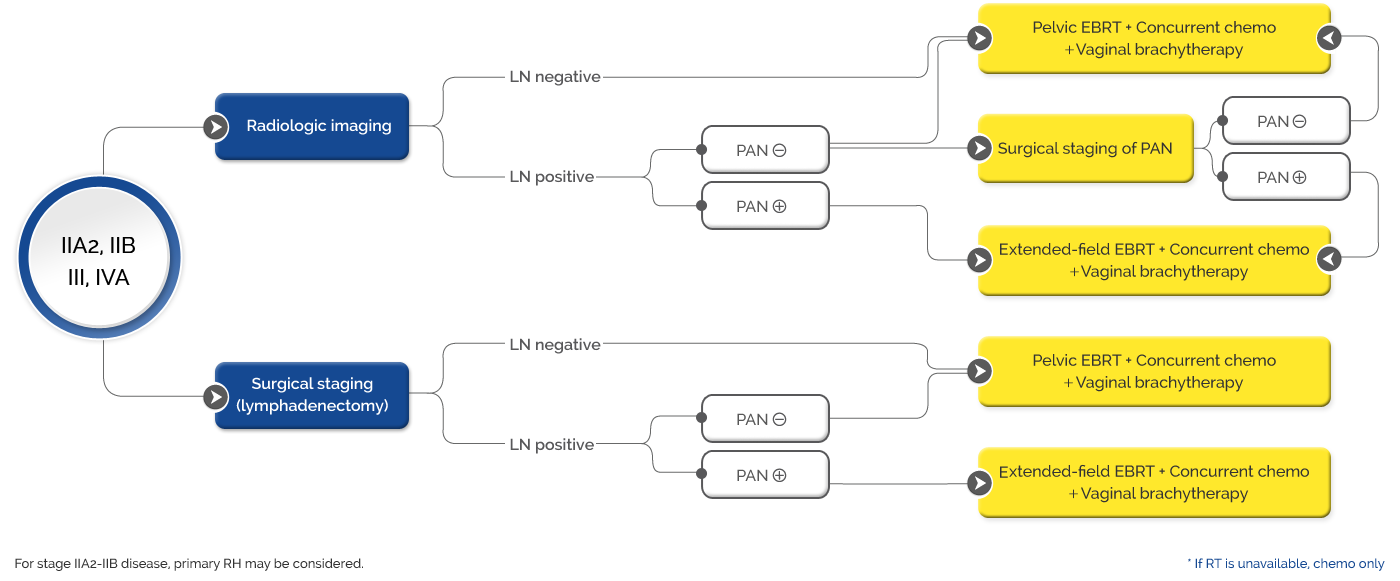
Newly diagnosed high-risk (FIGO 2014 stage 1B2-2B with LN-positive disease, and stage 3-4A with and without LN-positive disease) locally advanced cervical cancer